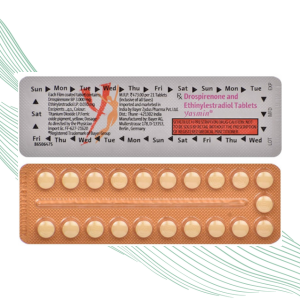
• Composition: Each film coated tablets contains: Drospirenone BP 3.000 mg. Ethinylestradiol IP 0.030 mg. Excipients q.s.
• This product will have minimum 3 months expiry at the time of order dispatch.
• Dosage: As directed by the Physician.
• Colours: Titanium dioxide IP. Ferric oxide pigment, yellow.
• Storage Instructions: Store below 25°C. Protect from moisture.
• Marketed by: Bayer Zydus Pharma Pvt Ltd
• Schedule H drug: Not be sold by retail without the prescription of a Registered Medical Practitioner.






Reviews
There are no reviews yet.